
It is not uncommon to see various medications used to treat Inflammatory Bowel Disease (IBD) described as “chemotherapy”. However, there appears to be a lot of confusion about what “chemotherapy” means. Misconceptions regarding chemotherapy and IBD have led to a significant amount of fear-mongering and disinformation regarding well studied medications. As a result, some people with IBD may be making treatment decisions based on faulty information and fear, rather than the best available evidence and careful consideration with a qualified gastroenterologist.
The term “chemotherapy” is being used ambiguously or inappropriately by many in and around the IBD community. What’s most troubling, though, is that some very high-profile health information websites have been spreading uncorroborated and inaccurate information about chemotherapy in IBD for years without any apparent sense of accountability. These sites are often the first resources patients come across when they search for information online, leading to many more people developing misconceptions and fear about the treatments used in IBD.
The objective of this article is to dispel some common misconceptions about the idea of chemotherapy in IBD. The objective of this article is definitely not to provide a detailed overview of the use of chemotherapy to treat cancer. Nor is it to provide a complete overview of the use of any therapies used to treat IBD. It’s not necessary to read every section to understand the most important points, which are highlighted in the summary table at the end. However, I encourage readers to not simply accept my conclusions, but to evaluate the information presented and discuss it with their gastroenterologists.
Table of Contents
What Does “Chemotherapy” Mean?
If you come across a discussion of chemotherapy in IBD, you will likely see “chemotherapy” defined as either “the treatment of disease using chemicals” or a type of cancer treatment (or some combination of the two). Despite its prevalent use, the first definition, “the treatment of disease using chemicals”, is not correct. If chemotherapy simply referred to “the treatment of disease using chemicals”, then the vast majority of medications could be called chemotherapy drugs — over 90% of drugs on the market are small-molecule drugs made through chemical synthesis (as opposed to biologics). 1http://pharma.bayer.com/en/innovation-partnering/technologies-and-trends/small-and-large-molecules/ 2http://link.springer.com/chapter/10.1007%2F978-1-4614-6916-2_2 3https://link.springer.com/chapter/10.1007%2F978-1-4614-6916-2_2 Even over-the-counter medications like aspirin would be considered chemotherapy if it was just about treating something with chemicals. That’s clearly not specific enough to be of much use.

Time for some history, dudes!
The meaning of words can and often do change over time. It’s reasonable for people to assume that “chemotherapy” was originally intended to mean the very literal “treatment of disease using chemicals”. This doesn’t do the word justice, though; the original meaning, as coined by Dr. Paul Ehrlich, was more nuanced than that. There are some who cling to this idea of what chemotherapy means out of an innocent misunderstanding. However, there are also those who fear-monger by characterizing certain treatments as being made of scary chemicals rather than some alternative they deem to be more natural. In reality, those products marketed as “natural” are fundamentally composed of chemicals as well, which not only highlights the absurdity of snake oil peddlers’ claims, but also the importance of education to resisting misinformation and fear-mongering. It’s important to not be misled by those who use the “chemotherapy” label or any mention of “chemicals” to elicit fear. Understanding the origin of the word “chemotherapy” can help one to understand just how ridiculous and misinformed these attempts to fear-monger are.

The modern usage of “chemotherapy” is almost universally referring to a type of cancer treatment. This is, unfortunately, where much of the fear and apprehension around this term comes from. The word “chemotherapy” evokes whatever associations people have with cancer and the therapies used to treat them. Once again, there are also those who deliberately fear monger by misusing the word “chemotherapy” to describe IBD treatments. While there is certainly a lot of misinformation regarding the use of chemotherapy for cancer, it becomes even more complicated when the term is applied (or misapplied) to IBD treatments. It’s important to understand what “chemotherapy” really refers to so that decisions made regarding treatment options for IBD are made using factual, useful information. Otherwise, decisions may instead be made based on misunderstandings or an aversion to the word “chemotherapy”.
Chemotherapia Specifica – The Original Meaning of “Chemotherapy”

Dr. Paul Ehrlich: Chemotherapy pioneer (1854-1915)
The term “chemotherapy” was coined in 1906 by Dr. Paul Ehrlich, a German-Jewish physician and scientist. 4https://www.nobelprize.org/prizes/medicine/1908/ehrlich/facts/ The literal meaning of “chemotherapy” is the treatment of disease with chemicals (“chemo-“, referring to chemicals, and “-therapy” referring to a disease therapy/treatment), but there is more to the word as Dr. Ehrlich defined it.
The use of chemicals to treat disease was not a new concept in Dr. Ehrlich’s time. Ehrlich himself once said “. . . from the very origin of the art of healing, chemotherapy has been in existence, since almost all of the medicaments that we employ are chemical.” However, he did not consider the approaches to treating disease with chemicals before his time to be the same as his idea of “chemotherapy”. The passage from Ehrlich continued with “on the other hand, experimental chemotherapy could only develop in a fruitful manner in modern times as a result of all the pioneer work.” The word “experimental” is significant here, as it indicates a much more distinct meaning than simply the use of chemicals to treat disease.
While Ehrlich sometimes used “chemotherapy” to refer to the general use of chemicals to treat disease, he typically used it to refer to a new and more sophisticated approach to the treatment of disease, of which he was a pioneer. According to chemist and medical historian Dr. John Parascandola, Ehrlich often qualified “chemotherapy” with “specific” or “experimental” to make clear its intended meaning. On account of this, German pharmacologist Hans Herken, in his 1976 address on Ehrlich’s chemotherapy at the Weizmann Institute of Science, stated that “the term ‘chemotherapy’ introduced by Paul Ehrlich is incomplete without the adjective ‘specifica’, which was also added by him.”5http://www.paul-ehrlich.de/Links/E016827665.pdf
When Dr. Ehrlich addressed the Berlin Medical Association of 1907, he described the philosophy of his work as the search for chemotherapia specifica: 6https://insights.ovid.com/american-medical-sciences/ajmeds/1967/12/000/fortschritte-der-arzneimittelforschung-progress/65/00000441 7https://books.google.com/books?id=Y8HzBwAAQBAJ&pg=PA390&dq=Progress+in+Drug+Research,+ehrlich&hl=en&sa=X&ved=0ahUKEwiC2rqskZneAhUFxoMKHYi4CfMQ6AEIKTAA#v=onepage&q=what%20we%20are%20seeking%20is%20chemotherapia%20specifica&f=false
Ehrlich based his drug development work around the idea that certain substances could selectively affect certain cells, tissues, or pathogens, via distinct receptors, without affecting others. Ehrlich came upon this idea of chemical specificity/selectivity early in his career while working with dyes which were found to colorize certain tissues and cells without affecting others. 8https://nyaspubs.onlinelibrary.wiley.com/doi/abs/10.1111/j.1749-6632.1954.tb45931.x He saw the potential for this same sort of specificity to be employed in the treatment of disease. 9https://www.karger.com/Article/Pdf/92200 10https://ac.els-cdn.com/B9780080090566500051/3-s2.0-B9780080090566500051-main.pdf?_tid=b454c467-b577-4df5-a721-329f040faa5d&acdnat=1552678448_fc112848f9e57ba5c964f91f2df21be9 The ideal chemotherapy specifica treatment that Ehrlich strove to develop, he described as Zauberkugeln – meaning “magic bullet”, another of his coined terms. 11https://jbuon.com/pdfs/9-4/JBUON-9-4-485.pdf
As for the qualifier “experimental”, Dr. Ehlrich saw chemotherapy as one aspect of experimental therapeutics, a field he considered a separate entity from the prevailing field of pharmacology. While pharmacology had developed a number of useful and beneficial drugs, Ehrlich argued that it had not developed any drugs that could provide specific cures. 12https://books.google.com/books/about/The_Collected_Papers_of_Paul_Ehrlich.html?id=3Bb-BAAAQBAJ In pharmacology at the time, medicines were primarily tested in healthy individuals and tissues. Dr. Ehrlich believed that experimental therapies should be tested in models that reflected the disease to be treated. To this end, he was a pioneer in the use of animal models of disease to systematically develop and screen new drugs. 13https://www.karger.com/Article/FullText/443526
It’s important to know that Ehrlich’s chemotherapy was primarily concerned with curing infectious diseases, which were the leading causes of death in his time. 14https://www.cdc.gov/mmwr/preview/mmwrhtml/mm4829a1.htm He initially described the mission of his institute of chemotherapy as follows: “Here we shall be concerned with the problem of curing organisms infected by certain parasites in such a way that the parasites are exterminated within the living organism, so that the organism is disinfected .. . by the use of substances which have had their origin in the chemist’s retort. Thus, the task of the new institute will be a specific chemotherapy of infectious diseases.”15https://academic.oup.com/jhmas/article-abstract/XXXVI/1/19/791052
This sounds very different from the modern idea of chemotherapy, which is so closely associated with cancer. Ehrlich did work towards applying chemotherapy to cancer as well. However, he saw cancer as analogous to a parasitic infection. 16https://ac.els-cdn.com/B9780080090566500051/3-s2.0-B9780080090566500051-main.pdf?_tid=b454c467-b577-4df5-a721-329f040faa5d&acdnat=1552678448_fc112848f9e57ba5c964f91f2df21be9 17https://nyaspubs.onlinelibrary.wiley.com/doi/pdf/10.1111/j.1749-6632.1954.tb45931.x He hoped to apply the idea of chemotherapia specifica to treating cancer in much the same way he was working to apply it to bacterial infections. Eventually, Ehrlich’s idea of chemotherapy was supplanted by the types of cancer chemotherapies one might think of today. In his 1964 article Historical Perspectives on Chemotherapy, Dr. E.K. Marshall, former head of The Department of Pharmacology and Experimental Therapeutics at John Hopkins Medicine, said:
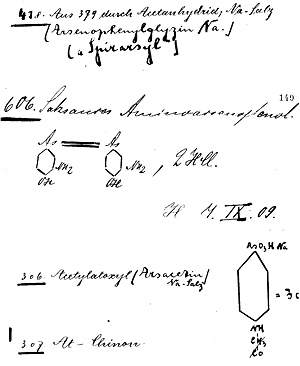
Entries from Dr. Ehrlich’s laboratory notebook regarding the development of compound 606. Image source: The Rockefeller University Hospital.
Dr. Ehrlich had significant success in the area of infectious disease. For instance, by 1909 he had contributed to the first diagnostic test for syphillis, and devised the first effective treatment for syphillis and other diseases caused by Treponema bacteria. This treatment, arsphenamine (trade name Salvarsan, meaning “the arsenic that saves”), was a direct product of his approach to specific and experimental chemotherapy. 19https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2790789/ In Ehrlich’s laboratory, arsphenamine was also referred to as compound 606; Ehlrich and his team (notably Dr. Sahachiro Hata) synthesized more than 1000 variations of an arsenic compound in their pursuit of an effective treatment for syphillis, and it was the 606th compound that proved to be the most effective. This compound was eventually improved upon by the 914th compound, which was dubbed neoarsphenamine. 20https://www.nobelprize.org/prizes/medicine/1908/ehrlich/biographical/ Arsphenamine and neoarsphenamine remained in use for many years, until they were largely replaced by Penicillin in the late 1940s. 21http://pubs.acs.org/cen/coverstory/83/8325/8325salvarsan.html 22http://centennial.rucares.org/index.php?page=Chemotherapy
The development, screening, and testing procedures Ehlrich and his team utilized laid the foundation for modern drug development practices. 23https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2790789/
While Dr. Ehrlich had ground-breaking successes in the treatment of certain infectious diseases, he was apparently less confident about the potential for chemotherapy to treat cancer 24http://cancerres.aacrjournals.org/content/68/21/8643.full 25https://onlinelibrary.wiley.com/doi/pdf/10.1002/1097-0142%28197010%2926%3A4%3C737%3A%3AAID-CNCR2820260402%3E3.0.CO%3B2-T Whatever level of optimism Ehrlich had about treating cancer with chemotherapy, the majority of the medical community was generally not optimistic. This did not really change until well over half a century after Dr. Ehrlich coined the term “chemotherapy”, following some major successes of chemotherapy in the treatment of acute childhood leukemia and advanced Hodgkin’s disease. Until then, surgery and radiotherapy were the dominant treatments for cancer. 26http://cancerres.aacrjournals.org/content/68/21/8643.full 27https://sciencebasedmedicine.org/chemotherapy-doesnt-work-not-so-fast-a-lesson-from-history/
Although Dr. Ehrlich died decades before chemotherapy became a viable treatment for cancer, his contributions to cancer research, drug development, and medicine in general, are substantial. His approach to drug development and his idea that drugs could be developed to target specific cells and tissues was profoundly influential. 28https://www.karger.com/Article/FullText/443526 His use of animal models to screen large numbers of compounds for their potential to treat specific conditions, and his work with transplantable tumors, were both very important to early cancer research. A major focus of the early decades of cancer chemotherapy research was the development of transplantable tumors and model development. 29http://cancerres.aacrjournals.org/content/68/21/8643.full In addition, Ehrlich is considered the “father of alkylating agents” – which were subsequently found to be the first effective chemotherapies for cancer. 30http://muse.jhu.edu/article/405762
Dr. Ehrlich was ahead of his time in many ways, and his influence on medicine can still be seen to this day. Many consider the development of monoclonal antibodies to be at least a partial realization of Ehrlich’s ideal Zauberkugeln (“magic bullet”). 31https://books.google.com/books/about/Love_and_Science.html?id=CycrCQAAQBAJ&printsec=frontcover&source=kp_read_button#v=onepage&q&f=false He is considered a founding figure in immunology, and was awarded the 1908 Nobel Prize in medicine for his contributions to that field. 32https://www.karger.com/Article/FullText/443526 In addition, he is considered the founder of modern hematology; many laboratory scientists and pathologists still regularly make use of his concepts and techniques. 33https://www.hematology.org/Thehematologist/Profiles/4513.aspx Incredibly, this is only scratching the surface of his contributions to medicine. This section of the article was never intended to be as long as it is, but Ehrlich’s story is too fascinating to completely skim over. Although his name is not well known now outside of the medical field, Dr. Ehrlich was more well known in decades past. The development of arsphenamine was such a significant event that the story was made into a movie 30 years later. “Dr. Ehrlich’s Magic Bullet” (1940) starred Edward G. Robinson as Dr. Ehrlich and tells the tale, in dramatic fashion, of the development of the first effective medical treatment for syphillis. 34https://www.imdb.com/title/tt0032413/ The original trailer describes Dr. Ehrlich as “the rebel genius who defied the world”. Check it out:

“Chemotherapy” is still used to refer to antimicrobial treatments in the academic and medical world.
It should be clear that “the treatment of disease using chemicals” is not sufficient to describe the original, intended meaning of “chemotherapy”. Regardless, the modern definition of “chemotherapy” nearly universally refers to certain types of drugs used to treat cancer. However, it’s worth pointing out that the original concept of chemotherapy as a treatment for infectious diseases is still in use as well.
The context that one is most likely to see the word “chemotherapy” correctly applied to nowadays other than cancer is in antimicrobial treatments, such as those used to treat bacterial infections like tuberculosis. This can be referred to as antimicrobial chemotherapy, or more specific names may be used for particular treatments, such as antibacterial chemotherapy or antiviral chemotherapy. Although the general public isn’t likely to use these terms, chemotherapy of infectious diseases, as Dr. Ehrlich originally used the term, is still in use in the medical and academic world.
The Modern Definition of “Chemotherapy”
According to surgical oncologist David Gorski, MD, PhD, “these days the term ‘chemotherapy’ is rarely used to describe anything other than the cytotoxic chemotherapy of cancer that in the popular mind causes so many horrific side effects.” 35https://www.sciencebasedmedicine.org/chemotherapy-doesnt-work-not-so-fast-a-lesson-from-history/
 Indeed, the U.S.’s National Library of Medicine encyclopedia entry for chemotherapy says: “The term chemotherapy is used to describe cancer-killing drugs.” 36https://www.nlm.nih.gov/medlineplus/ency/article/002324.htm
Indeed, the U.S.’s National Library of Medicine encyclopedia entry for chemotherapy says: “The term chemotherapy is used to describe cancer-killing drugs.” 36https://www.nlm.nih.gov/medlineplus/ency/article/002324.htm
Likewise, the U.K.’s National Health Service describes chemotherapy as “a type of cancer treatment, with medicine used to kill cancer cells.” 37http://www.nhs.uk/conditions/Chemotherapy/Pages/Definition.aspx
The National Cancer Institute describes chemotherapy as a “treatment that uses drugs to stop the growth of cancer cells, either by killing the cells or by stopping them from dividing.” 38https://www.cancer.gov/publications/dictionaries/cancer-terms/def/chemotherapy
The modern usage of “chemotherapy” typically refers to, at minimum, a type of medicine used to treat cancer. More specifically, as the National Cancer Institute’s definition reflects, the term chemotherapy is reserved for medications that are cytotoxic (kill cells) or cytostatic (stop cells from dividing). There are cancer therapies that function in ways beyond cytotoxic/cytostatic activity, and so are categorized separately from chemotherapy. The National Cancer Institute, for instance, lists immunotherapy, targeted therapy, hormone therapy, stem cell transplant, and chemotherapy as the major categories of non-surgical and non-radiation treatments used for malignant conditions. 39https://www.cancer.gov/about-cancer/treatment/types
If chemotherapy refers to a cytotoxic or cytostatic cancer therapy, then that can help to explain the fear and apprehension associated with the term. According to Dr. Gorski, “When people think of chemotherapy, they think of hair falling out, nausea and vomiting, fatigue, and death.” 40https://www.sciencebasedmedicine.org/chemotherapy-doesnt-work-not-so-fast-a-lesson-from-history/ Of course, there’s certainly evidence supporting the potential for adverse effects from cancer chemotherapy. 41http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3505531/ 42http://annonc.oxfordjournals.org/content/early/2011/02/02/annonc.mdq683.full 43http://www.sciencedirect.com/science/article/pii/S0304395914004436 That said, many people are likely unfamiliar with the medical advances made in recent decades, and so conceptions of chemotherapy may be based on outdated impressions or incorrect information. 44https://www.cancer.gov/about-cancer/understanding/statistics Furthermore, there are hundreds of types of cancer and many types of chemotherapy drugs and regimens, with many varied experiences among the people receiving treatment. 45http://www.oncologynurseadvisor.com/chemotherapy-regimens/section/2171/ On top of that, it is often difficult to separate the effects of cancer from the drugs used to treat them. 46https://www.sciencebasedmedicine.org/chemotherapy-doesnt-work-not-so-fast-a-lesson-from-history/ However, the important thing to understand here is that the word “chemotherapy” elicits strong feelings in many people, including those with IBD.
The issue with applying the “chemotherapy” label to IBD treatments isn’t due to chemotherapy being bad; on the contrary, it’s a very important type of treatment for very difficult diseases and the world is absolutely better off with them available. There is a lot of misinformation and fear-mongering regarding the medical treatment of cancer, and too often it leads to people dying of curable diseases. 47https://academic.oup.com/jnci/article/110/1/121/4064136 However, that’s a separate issue from the point of this article. The issue with applying the “chemotherapy” label to IBD treatments is due to the associations people make with the word. Studies have shown that people often have specific expectations about the adverse effects of chemotherapy treatments for cancer. 48https://www.ncbi.nlm.nih.gov/pubmed/27551051 49https://www.jpsmjournal.com/article/S0885-3924(12)00074-7/pdf 50http://onlinelibrary.wiley.com/doi/10.1002/cncr.20423/abstract Some common expectations are fatigue, nausea, sleep disturbance, weight loss, hair loss, and skin problems. 51http://onlinelibrary.wiley.com/doi/10.1002/cncr.20423/abstract Interestingly, one study found that people without cancer report perceiving “most chemotherapy-associated side effects as having greater impact on the quality of life than did cancer patients who had received chemotherapy”. 52http://www.ncbi.nlm.nih.gov/pubmed/10352062 Right or wrong, I think this helps illustrate how a label like “chemotherapy” can cause people with IBD to anticipate severe issues from their medications. This can lead to people making decisions about treatments based on fear, rather than good information and careful consideration with their physicians.
—————————-
There are several true cancer chemotherapy drugs that are also being used to treat IBD. However, there are some very significant differences between the way these drugs are used when treating cancer and how they’re used when treating IBD.
The Use of Chemotherapy Agents in IBD
Methotrexate, mercaptopurine, and azathioprine are chemotherapy agents that are also used in the treatment of IBD. All of these medications had a history of use in cancer before it was discovered they could also be helpful in the treatment of autoimmune and immune-mediated conditions like IBD. 53http://www.chemheritage.org/discover/online-resources/chemistry-in-history/themes/pharmaceuticals/restoring-and-regulating-the-bodys-biochemistry/hitchings–elion.aspx 54http://tih.sagepub.com/content/2/2/1.extract 55https://www.ncbi.nlm.nih.gov/pmc/articles/PMC152947/
Chemotherapy Drugs are Immunomodulators in IBD
While methotrexate, mercaptopurine, and azathioprine may be chemotherapy agents, when they are used to treat IBD they are classified as immunomodulators. An immunomodulator is, simply put, a drug that modifies (modulates) the way the immune system functions. 56https://www.crohnscolitisfoundation.org/assets/pdfs/immunomodulators.pdf When these drugs are used in IBD, they are used with the intent of producing an immune-modulating effect, not a chemotherapeutic one, and the dosage used in IBD is associated more with an immune-modulating effect than a chemotherapeutic one. Furthermore, they aren’t used as part of a chemotherapy regimen as they would be in cancer; they are used as part of an IBD treatment plan. They are described as immunomodulators in Gastroenterology clinical practice guidelines and in patient-targeted information from organizations like The Crohn’s & Colitis Foundation. 57https://www.gastrojournal.org/article/S0016-5085(13)01521-7/fulltext 58https://www.gastrojournal.org/article/S0016-5085(06)00073-4/fulltext 59http://www.crohnscolitisfoundation.org/resources/immunomodulators.html
Immunomodulators are commonly used in combination with biologics to treat IBD. The reason for this is that they often work better in combination with biologics compared to on their own, and they may help biologics to be more effective and maintain that effectiveness. Biologic therapies are made of big, complex molecules that our bodies don’t produce themselves, which means one’s immune systems may identify them as foreign and develop its own antibodies to remove them. Immunomodulators may help prevent the formation of these anti-drug antibodies, thereby allowing the biologic to work more effectively and for longer. 60https://journals.lww.com/ajg/Fulltext/2019/03000/ACG_Clinical_Guideline__Ulcerative_Colitis_in.10.aspx 61https://journals.lww.com/ajg/Fulltext/2018/04000/ACG_Clinical_Guideline__Management_of_Crohn_s.10.aspx 62https://www.ncbi.nlm.nih.gov/pubmed/30824404 63https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5421108/ The rate at which biologics are cleared from the body is also associated with the amount of inflammation present. More inflammation is associated with increased clearance, which means the biologic has less time to work in the body. Immunomodulators may simply help reduce inflammation, which decreases the rate at which biologics are cleared from the body, thereby increasing the chance of the biologic working. 64https://www.nejm.org/doi/full/10.1056/NEJMoa0904492 65https://academic.oup.com/rheumatology/article/55/2/210/1822054 This is a really simplified overview, but it’s important to understand the general concept and reasoning for the use of immunomodulator + biologic combination therapy in IBD.
Chemotherapy Drugs, But Not Chemotherapy Regimens
In the context of cancer, methotrexate, mercaptopurine, and azathioprine are chemotherapy drugs. In addition, when used in the treatment of cancer, they are used as part of a chemotherapy regimen. A chemotherapy regimen is a specific plan for cancer chemotherapy treatment that defines the drugs to be used, the doses to be taken, the frequency each drug should be taken, the duration each drug should be taken, any additional medications needed to manage toxicities, and so forth. 66http://www.oncologynurseadvisor.com/chemotherapy-regimens/section/2171/ 67https://lymphoma-action.org.uk/about-lymphoma-treatment-lymphoma-chemotherapy/chemotherapy-regimens-lymphoma Chemotherapy regimens typically involve a combination of drugs that may include conventional chemotherapy agents along with other therapies, such as targeted antibodies like rituximab (Rituxan). 68https://lymphoma-action.org.uk/about-lymphoma-treatment-lymphoma-chemotherapy/chemotherapy-regimens-lymphoma In addition, there may be multiple phases of treatment with different chemotherapy schedules depending on the type of cancer and goal of treatment. As part of a chemotherapy regimen, methotrexate, mercaptopurine, and azathioprine may be used alongside other chemotherapy and cancer-targeting drugs. In fact, methotrexate and mercaptopurine are commonly used alongside each other, such as in the treatment of acute lymphoblastic leukemia. 69http://journals.lww.com/jpho-online/Fulltext/2014/10000/Mercaptopurine_Methotrexate_Maintenance_Therapy_of.1.aspx
When used to treat IBD, methotrexate, mercaptopurine, and azathioprine are immunomodulators, and are used as part of an IBD treatment plan rather than a chemotherapy regimen. In short, the general goal of an IBD treatment plan is to regulate the dysregulated immune response which is leading to inflammation, achieve remission (meaning there is no active disease/inflammation), manage symptoms, and avoid relapses (a return of active disease/inflammation). 70http://www.uchospitals.edu/specialties/gi/ibd/treatment.html 71https://journals.lww.com/ajg/Fulltext/2019/03000/ACG_Clinical_Guideline__Ulcerative_Colitis_in.10.aspx 72https://journals.lww.com/ajg/Fulltext/2018/04000/ACG_Clinical_Guideline__Management_of_Crohn_s.10.aspx The specific treatments used and other details can change over time to fit the patient’s needs (e.g. when trying to induce remission vs. when trying to maintain it), but that’s the general, overall gist of it.
—————————-
While it’s not inaccurate to say that chemotherapy agents are used to treat IBD, it is also not very helpful to understanding how these drugs work and why they’re used in IBD. To reiterate, the issue then is that people may make false assumptions about these IBD treatments based only on their impression of the word “chemotherapy”. Therefore, it’s important to understand that in the context of IBD, chemotherapy drugs are immunodulators, and they’re used as part of an IBD treatment plan. Read on for more specific information about methotrexate, mercaptopurine, and azathioprine.
Methotrexate, Mercaptopurine, & Azathioprine: A Brief Overview
Methotrexate
Methotrexate
Methotrexate (trade names: Rheumatrex, Trexall, Otrexup, Rasuvo) is a folate analogue and antagonist. This basically means that, because its chemical structure is so similar to folate (analogue), it interferes with folate-dependent processes in the body (antagonist). It is also referred to as an antimetabolite, since it interferes with metabolic pathways associated with folate. 73http://chemoth.com/types/antimetabolites 74https://www.cancer.gov/publications/dictionaries/cancer-terms/def/folate-antagonist It is used as chemotherapy in the treatment of various cancers, and as an immunomodulator in the treatment of immune-mediated diseases like IBD.
The Dosage of Methotrexate in Cancer vs. IBD
According to the University of North Carolina Medicine’s Gastroenterology department:
When methotrexate is used in the treatment of IBD, it is considered “low dose” therapy. Low dose methrotrexate refers to body surface area-based doses of less than or equal to 50 mg/m2, but the doses used in IBD are actually at the very low end of that. Methotrexate is typically used at 5-25 mg/week for IBD, with a common dosage being 15 mg per week; 76https://www.sciencedirect.com/science/article/pii/S0016508518349333 for a 6 ft tall, 180 lb person, that amounts to less than 8 mg/m2 using body surface area dosing. 77https://www.ncbi.nlm.nih.gov/pubmed/21199463 78https://reference.medscape.com/calculator/bsa-dosing When methotrexate is used for cancer, it most commonly falls into the category of “high dose” therapy. 79https://www.healio.com/hematology-oncology/leukemia/news/print/hemonc-today/%7Bca58b498-9a79-464f-a89b-2641c2a71aa1%7D/high-dose-methotrexate-therapy-focus-on-glucarpidase 80http://theoncologist.alphamedpress.org/content/early/2016/08/05/theoncologist.2015-0164.full.pdf 81https://www.stjude.org/treatment/patient-resources/caregiver-resources/medicines/a-z-list-of-medicines/leucovorin-with-high-dose-methotrexate-hdmtx.html High dose methotrexate typically refers to body surface area based-doses of greater than or equal to 500 mg/m2. A common single dose for cancer is around 1500mg, although it can be used at much greater doses. For instance, intravenous (IV) infusions of around 5,000 mg/m2 are used in some chemotherapy regimens; for someone 6 feet tall and 180 lbs, this would come out to around 10,000 mg. 82http://www.uptodate.com/contents/therapeutic-use-and-toxicity-of-high-dose-methotrexate 83https://www.uptodate.com/contents/major-side-effects-of-low-dose-methotrexate 84https://www.cancertherapyadvisor.com/home/cancer-topics/hematologic-cancers/leukemia-treatment-regimens-acute-lymphoblastic-leukemia-all/ In addition to differences in dosage, methotrexate may also be administered differently when used as chemotherapy. For instance, while methotrexate is only administered as an oral pill or subcutaneous (under the skin) injection in IBD, it is commonly administered as an IV infusion or intrathecal (into the spinal column) injection for cancer. 85https://emedicine.medscape.com/article/2004705-overview
Low dose methotrexate has also been studied for use alongside cancer immunotherapies due to its immunomodulating effect (and not the cytotoxic effect seen in higher doses). When low dose methotrexate is used in cancer, it is often described in medical literature as “noncytotoxic concentrations”. 86https://onlinelibrary.wiley.com/doi/full/10.1002/ijc.27801 87https://www.ncbi.nlm.nih.gov/pubmed/21290210 88https://www.ncbi.nlm.nih.gov/pubmed/19591684 The prolonged use of low doses of chemotherapy agents in the treatment of cancer is often referred to as metronomic chemotherapy or low-dose chemotherapy. 89https://www.nature.com/articles/nrclinonc.2016.64 90https://www.cancer.gov/publications/dictionaries/cancer-terms/def/metronomic-therapy 91https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4156829/ From a 2017 Trends in Cancer paper on metronomic chemotherapy, the authors state that “the mechanism of action of a given anticancer agent can significantly differ when it is given metronomically compared with when it is administered following an MTD [Maxium Tolerated Dose] schedule.” 92https://www.sciencedirect.com/science/article/abs/pii/S2405803317300663 This means that when methotrexate, or other chemotherapy agents, are used metronomically in the treatment of cancer, any potential benefit or effect may be due to a mechanism of action other than the cytotoxic one expected with higher doses. 93https://www.ncbi.nlm.nih.gov/pubmed/11863115 However, some other studies suggest that low, noncytotoxic concentrations of methotrexate are not beneficial in the treatment of cancer. 94https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5323031/ 95https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3111194/ 96https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5035120/
How Methotrexate Works in Cancer vs. IBD (A Very Basic Introduction)
There are still new things being discovered about the way methotrexate works. Nevertheless, there are some notable differences between the way methotrexate has been found to work in cancer compared to how it works in IBD. The following is a simplified look at some of the differences.
As mentioned previously, methotrexate is a folate analogue, which means its chemical structure is very similar to folate, but with some key differences that lead to an interference in normal biochemical activities. Folate is a B vitamin that is an important and necessary component of various processes in the body, including the production of DNA and RNA, and cell division. 97https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/ As methotrexate is a folate analogue, the body essentially mistakes it for true folate and incorporates it into areas where folate is used. By taking the place of folate, methotrexate interferes with the metabolism of folate and with folate-dependent processes. For instance, high dose methotrexate, as used in cancer, suppresses the action of the folate-dependent enzyme dihydrofolate reductase. This enzyme is needed to produce purine and pyrimidines, components of both DNA and RNA. When DNA and RNA production is inhibited, the proliferation of new cells is also inhibited. This is an important strategy in malignant conditions, such as leukemia, where there is an over-proliferation of abnormal cells. 98https://theoncologist.alphamedpress.org/content/4/4/340.full 99https://www.cancer.gov/publications/dictionaries/cancer-terms/def/leukemia 100https://www.cancer.gov/research/progress/discovery/methotrexate Methotrexate is primarily taken up by cells that are rapidly dividing, such as leukemia cells. 101https://onlinelibrary.wiley.com/doi/full/10.1002/art.20278 Furthermore, the cytotoxic components of methotrexate bind to folate receptors in the body less readily than actual folate, which may help explain the need for high doses of methotrexate to produce cytotoxic effects. 102https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3033035/ 103https://pdfs.semanticscholar.org/e9ba/a4c961f20f33d9483810acf3382f9da34776.pdf 104https://www.ncbi.nlm.nih.gov/pubmed/2552922 105https://www.exagen.com/wp-content/uploads/Toward-a-Better-Understanding-of-Methotrexate.pdf
Low dose methotrexate, as used in IBD, is not associated with the same anti-proliferative effect. 106https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6087553/ There are a number of ways that low dose methotrexate is thought to have an anti-inflammatory effect without the cytotoxicity seen in higher doses. One of the primary ways is through adenosine-mediated immune-modulation. 107https://www.ncbi.nlm.nih.gov/pubmed/17922664 Adenosine is a purine nucleoside that is involved in the regulation of inflammation. 108https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3619132/ Methotrexate is typically cleared very quickly from one’s blood (primarily through the kidneys). However, methotrexate polyglutamates (a metabolic product of methotrexate) can remain in cells for weeks. 109https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3769795/ One of the most pronounced effects of methotrexate polyglutamates in low dose methotrexate therapy is the suppression of metabolic pathways that break down adenosine. This leads to an increase in intracellular (within cells) and extracellular (outside of cells) adenosine and a reduction in inflammation. 110https://www.ncbi.nlm.nih.gov/pubmed/21199463 111https://www.ncbi.nlm.nih.gov/pmc/articles/PMC128935/ 112https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1798086/ 113https://arthritis-research.biomedcentral.com/articles/10.1186/ar419 There are receptors on the surface of cells (adenosine receptors) that, when bound to by adenosine, lead to decreased production of pro-inflammatory agents as well as increased production of anti-inflammatory ones. One example of a pro-inflammatory agent that is decreased is TNF, the same protein that is inhibited by biologic medications like infliximab (Remicade) and adalimumab (Humira). Another example is IL-12, one of the proteins that is inhibited by the biologic ustekinumab (Stelara). 114https://ard.bmj.com/content/60/8/729 115https://www.ncbi.nlm.nih.gov/pubmed/21199463 116https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3619132/
An interesting bit of trivia: It wasn’t known that methotrexate could play a role in the treatment of immune-mediated conditions like IBD until leukemia patients with coexistent autoimmune disease saw improvement in their autoimmune conditions while on high dose methotrexate for the leukemia. 117https://academic.oup.com/ecco-jcc/article/9/4/312/488446 118https://www.nejm.org/doi/full/10.1056/NEJM194806032382301 But again, high dose methotrexate is not indicated for IBD.
According to a 2010 paper by Herfarth, et al. on the use of methotrexate in ulcerative colitis, published in the Crohn’s & Colitis Foundation’s Inflammatory Bowel Diseases medical journal:
The bold text above (emphasis mine) is important. The anti-proliferative activity of low-dose methotrexate is minimal at the doses used in IBD. The effects of low-dose methotrexate are ultimately more anti-inflammatory than cytotoxic. Methotrexate may be a chemotherapy agent, but when given at the lower doses used in IBD, it is used as an immunomodulator.
Folic Acid Supplementation
When methotrexate is used to treat IBD, a folic acid supplement (folic acid is a synthetic form of folate) is prescribed alongside it. As methotrexate is a folate analogue, it interferes with folate-dependent processes and ultimately reduces the amount of folate in the body. By taking a prescribed folic acid supplement, IBD patients can avoid or significantly minimize any potential side effects of methotrexate. A common dose used here is 1 mg/day or 5 mg/week. 120https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4679484/ 121http://www.ncbi.nlm.nih.gov/pubmed/23728635 122http://www.medscape.com/viewarticle/588229 123http://metoject.ru/upload/file/Understanding%20the%20mechanisms%20of%20action%20of%20MTX.pdf 124https://www.uptodate.com/contents/overview-of-medical-management-of-high-risk-adult-patients-with-moderate-to-severe-crohn-disease 125http://www.med.umich.edu/ibd/education/medications.html
When methotrexate is used to treat a malignant condition, however, patients are generally advised to avoid ongoing folic acid supplementation as it may interfere with the (high dose) methotrexate’s cytotoxic cancer-killing effects. 126https://arthritis-research.biomedcentral.com/articles/10.1186/ar419 127http://chemocare.com/chemotherapy/drug-info/Methotrexate.aspx 128http://umm.edu/health/medical/altmed/supplement-interaction/possible-interactions-with-vitamin-b9-folic-acid 129http://www.cancer.org/cancer/gestationaltrophoblasticdisease/detailedguide/gestational-trophoblastic-disease-treating-chemotherapy Instead, cancer patients receiving high dose methotrexate are given folinic acid (a.k.a. leucovorin; a prescription-only vitamer of folic acid) “rescue” therapy following methotrexate treatment, to help protect normal cells from the toxic effects of high dose methotrexate. 130https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5153332/ 131http://www.cancer.org/cancer/gestationaltrophoblasticdisease/detailedguide/gestational-trophoblastic-disease-treating-chemotherapy
—————————-
The following table helps to summarize a lot of the important information needed to differentiate the mechanism of action and general use of high dose methotrexate with low dose methotrexate. It is from a 2010 paper by Malaviya et al., Low-dose and high-dose methotrexate are two different drugs in practical terms, published in the International Journal of Rheumatic Diseases. The focus of this paper was primarily on rheumatic conditions (hence no specific mention of IBD under indications of low dose methotrexate), but the information regarding low dose methotrexate (LD-MTX) applies to IBD as well. 132https://www.ncbi.nlm.nih.gov/pubmed/21199463
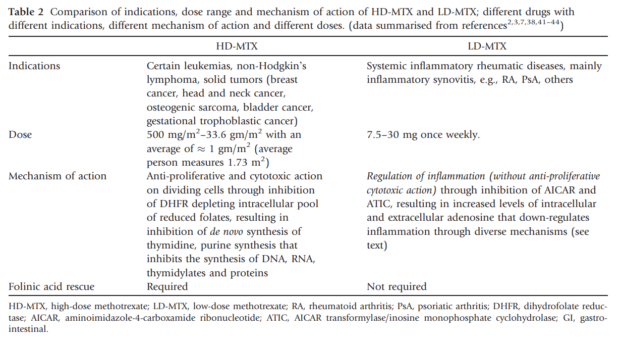
High dose vs low dose methotrexate. Table is from the 2010 paper by Malaviya et al., “Low-dose and high-dose methotrexate are two different drugs in practical terms”, published in the International Journal of Rheumatic Diseases. 133https://www.ncbi.nlm.nih.gov/pubmed/21199463
—————————————————————————————————
Thiopurines (Mercaptopurine & Azathioprine)

Mercaptopurine
Mercaptopurine aka 6-Mercaptopurine or 6-MP (trade names: Purinethol, Purixan) is a purine analogue and antagonist. This basically means that, because its chemical structure is so similar to purines (analogue), it is able to interfere with purine-dependent processes in the body (antagonist). It is also referred to as an antimetabolite, since it interferes with metabolic pathways associated with purines. 134http://chemoth.com/types/antimetabolites It is used to treat certain types of cancers, most notably acute lymphoblastic leukemia (ALL). It is also used in the treatment of some non-malignant conditions, such as Inflammatory Bowel Disease. 135https://www.nlm.nih.gov/medlineplus/druginfo/meds/a682653.html
Azathioprine (trade names: Imuran, Azasan) is a pro-drug of mercaptopurine, which means it is metabolized into mercaptopurine in the body. Azathioprine was originally developed in an attempt to slow the breakdown of mercaptopurine in the body, thereby protecting the anti-leukemic effect. 136https://science.sciencemag.org/content/244/4900/41.long
These two drugs are often referred to as thiopurines, which is another name for these types of purine antimetabolites. 137https://fg.bmj.com/content/9/1/10
Early on, mercaptopurine was discovered to be a ground-breaking treatment to prevent organ transplant rejection; azathioprine was subsequently found to be even better. The success of these drugs as immune-suppressants in the prevention of transplant rejection led to their use in the treatment of autoimmune and immune-mediated conditions like IBD. 138https://www.ncbi.nlm.nih.gov/pubmed/17970886 139http://science.sciencemag.org/content/244/4900/41 Outside of immune-mediated conditions, mercaptopurine is most known as a treatment for cancer, while azathioprine gained prominence as an anti-rejection treatment. That said, for the purpose of this article, much of the general information regarding mercaptopurine can be applied to azathioprine as well, so the name “thiopurines” may be used to refer to them collectively. 140https://link.springer.com/article/10.1007/BF01119794 141http://www.njmonline.nl/getpdf.php?id=1327
The Dosage of Thiopurines in Cancer vs. IBD
The dosage for mercaptopurine is generally higher when used for cancer than when used for IBD. For instance, while the maximum dosage for IBD commonly falls in the range of 0.75-1.5 mg/kg, it is commonly used at 1.5-5.0 mg/kg in some cancers. 142https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/009053s032lbl.pdf 143https://www.ncbi.nlm.nih.gov/pubmed/30044357 The doses used for azathioprine in the same patient are going to be higher than mercaptopurine in order for it to break down into a comparable amount of mercaptopurine in the body (azathioprine is 55% mercaptopurine by molecular weight, and 88% of it is converted to mercaptopurine in the body, so a conversion factor of 2.08 is used to convert mercaptopurine doses to azathioprine doses). 144http://reference.medscape.com/drug/purinethol-purixan-mercaptopurine-342094 145https://www.uptodate.com/contents/overview-of-azathioprine-and-mercaptopurine-use-in-inflammatory-bowel-disease?search=azathioprine&source=search_result&selectedTitle=2~148&usage_type=default&display_rank=1#H3912407284 146https://www.cghjournal.org/article/S1542-3565(04)00344-1/pdf 147https://gut.bmj.com/content/48/5/591
While thiopurines are only taken orally for IBD, intravenous (IV) mercaptopurine is also used in some cancer chemotherapy regimens. In these cases, doses of 1000 mg/m2 or greater, infused over 8 or more hours, may be used. 148https://ascopubs.org/doi/10.1200/jco.1993.11.9.1826 149https://www.ncbi.nlm.nih.gov/pubmed/9095207 In addition, when used to treat cancer, thiopurines are nearly always used alongside other chemotherapy agents, including methotrexate, which increases the cytotoxic effects that are important for successful cancer treatment. 150http://emedicine.medscape.com/article/2004705-overview 151http://journals.lww.com/jpho-online/Fulltext/2014/10000/Mercaptopurine_Methotrexate_Maintenance_Therapy_of.1.aspx 152https://www.uptodate.com/contents/mercaptopurine-drug-information?search=6-mp&source=panel_search_result&selectedTitle=1~121&usage_type=panel&kp_tab=drug_general&display_rank=1#F193232 153https://www.uptodate.com/contents/overview-of-the-treatment-of-acute-lymphoblastic-leukemia-in-children-and-adolescents 154https://link.springer.com/article/10.1007/s00280-014-2613-7 155https://www.cancertherapyadvisor.com/home/cancer-topics/hematologic-cancers/leukemia-treatment-regimens-acute-lymphoblastic-leukemia-all/ 156https://www.who.int/selection_medicines/committees/subcommittee/2/cytotoxic_review.pdf
How Thiopurines Work in Cancer vs. IBD (A Very Basic Introduction)
There are still new things being discovered about the way thiopurines work. Nevertheless, there are some notable differences between the way thiopurines have been found to work in cancer compared to how they work in IBD. The following is a simplified look at some of the differences.
As discussed in the previous section, thiopurines are generally dosed more aggressively in cancer treatment compared to IBD. Higher doses are important for producing the desired cytotoxic/antimetabolite effect in cancer treatment. For IBD treatment, the dosage is reflective of the desired immune-modulating effect. 157https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3971986/ 158http://www.jimmunol.org/content/170/10/4986.long 159https://www.ncbi.nlm.nih.gov/pubmed/30195449 160https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5107185/
An important difference between the use of mercaptopurine in cancer versus in IBD is the sort of treatment regimen it’s being used in. When used to treat cancer, thiopurines are nearly always used as part of a multi-drug chemotherapy regimen. For instance, in the treatment of ALL, mercaptopurine is typically combined with methotrexate and other chemotherapy drugs. Depending on the phase or goal of treatment, the doses used for these chemotherapy drugs can be very high. 161https://www.ucsfhealth.org/conditions/acute_lymphoblastic_leukemia/treatment.html 162http://www.purixan-us.com/resources/Package_Insert.pdf 163https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=40b09616-5bb1-4ef8-98cd-d87537254296&type=display 164https://www.cancertherapyadvisor.com/home/cancer-topics/hematologic-cancers/leukemia-treatment-regimens-acute-lymphoblastic-leukemia-all/ 165https://www.cancer.gov/types/leukemia/hp/child-all-treatment-pdq This is completely unlike the use of thiopurines in an IBD treatment plan. 166https://journals.lww.com/ajg/Fulltext/2019/03000/ACG_Clinical_Guideline__Ulcerative_Colitis_in.10.aspx 167https://journals.lww.com/ajg/Fulltext/2018/04000/ACG_Clinical_Guideline__Management_of_Crohn_s.10.aspx
While azathioprine is a prodrug of mercaptopurine (meaning it is metabolized or broken down into mercaptopurine in the body), mercaptopurine is itself a prodrug, as it breaks down into multiple metabolites once in the body. Some of these metabolites have particular importance in the treatment of cancer, while others are more important in the treatment of IBD.
Cancer: When there is a sufficient amount of certain metabolites present, a cytotoxic effect can be produced. 6-thioguanine nucleotide (6TGN) metabolites are incorporated into the DNA of rapidly dividing cells (e.g. leukemia cells) in place of normal DNA components, leading to errors which result in cytotoxicity. 168https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4737107/ 169https://www.ncbi.nlm.nih.gov/pubmed/19956952 In addition, some 6TGN metabolites are incorporated into RNA which interferes with protein synthesis, while metabolites such as thioinosine monophosphate (TIMP) and methyl thioinosine monophosphate (Me-TIMP) inhibit purine synthesis, further contributing to the cytotoxicity. 170https://www.ncbi.nlm.nih.gov/pubmed/19956952 171http://cancerres.aacrjournals.org/content/35/10/2872.long 172https://www.hindawi.com/journals/mi/2015/434825/ 173https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/009053s032lbl.pdf 174http://www.bccancer.bc.ca/drug-database-site/Drug%20Index/Mercaptopurine_monograph.pdf As mentioned, thiopurines primarily affect cells that are rapidly dividing, such as leukemia cells (and other blood cells). In addition, leukemia cells may have increased susceptibility to the antimetabolite effects of thiopurines compared to normal leukocytes (white blood cells). 175https://www.ncbi.nlm.nih.gov/pubmed/19648785/ 176https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4222610/
IBD: The immunomodulating effects of thiopurines seen in IBD can’t be explained by the cytotoxic mechanisms of action seen in cancer chemotherapy. 177https://www.cghjournal.org/article/S1542-3565(05)00697-X/pdf One of the most important ways thiopurines have been found to work in IBD is through the modulation/inhibition of a GTPase enzyme called Rac1 (Ras-related C3 botulinum toxin substrate 1). Normally, GTPase enzymes, like Rac1, bind to guanosine triphosphate (GTP). The thiopurine metabolite 6-thioguanine triphosphate (6-TGTP) competes with GTP for the same binding sites on Rac1. By out-competing for these spots, it prevents Rac1 from carrying out its normal functions. Rac1 plays an essential role in the preservation of activated immune cells in the gut (among other functions related to immune cells). These activated immune cells (T lymphocytes) contribute to the inflammation within the gut, such as by the release of pro-inflammatory cytokines like TNF. When 6-TGTP interferes with normal Rac1 activity, a “programmed cell death” signal (apoptotic signal) is sent to these activated immune cells. In short, this leads to a reduction in activated immune system cells in the gut, which ideally helps to reduce inflammation. 178https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2933759/ 179https://www.ncbi.nlm.nih.gov/pubmed/12697733/ 180https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5107185/ 181https://academic.oup.com/ibdjournal/article-abstract/20/9/1487/4579082
According to Dr. Markus Neurath, lead author of an important 2003 paper detailing the effect of thiopurines on Rac1:
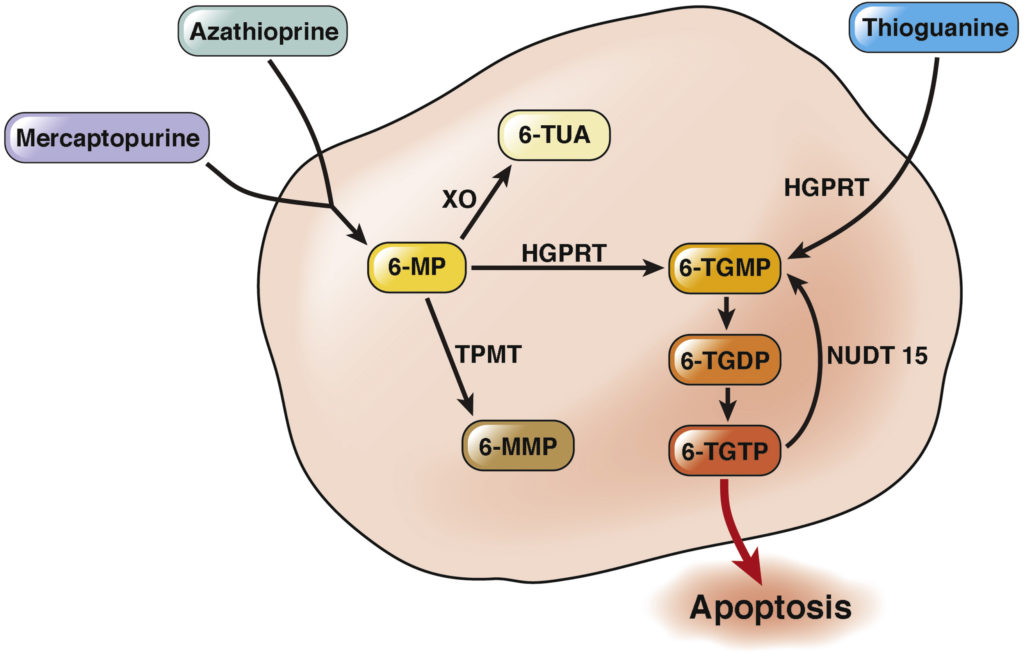
A “simplified thiopurine metabolism scheme” describing the function of thiopurines in IBD. From Dr. Nanne K.H. de Boer & The Thiopurine Working Group. 183https://www.gastrojournal.org/article/S0016-5085(18)35287-9/fulltext
IBD patients without active disease have shown decreased inflammatory Rac1 activity compared to patients with active disease. In addition, it has been shown that in IBD patients whose disease responds well to thiopurines, there is an associated reduction in Rac1 activity. 184https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5107185/ 185https://www.ncbi.nlm.nih.gov/pubmed/27465973/ Furthermore, successful treatment of IBD involving thiopurines has been associated with an increase in apoptotic T lymphocytes in the intestinal mucosa. 186https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1965586/ This essentially means that activated immune cells in the intestines have a shortened lifespan, so they have less chance to contribute to inflammation. 187https://www.ncbi.nlm.nih.gov/pmc/articles/PMC152932/
Bone Marrow Suppression
In the 2017 article by de Boer, et al., “Thiopurines in Inflammatory Bowel Disease: New Findings and Perspectives”, from the Journal of Crohn’s and Colitis, the authors state:
When acute lymphoblastic leukemia (ALL) is in remission, mercaptopurine is commonly used alongside methotrexate (and possibly other drugs) as part of a maintenance chemotherapy regimen. This sort of maintenance regimen in leukemia is sometimes referred to as myelosuppresion maintenance therapy – “myelo-” referring to the bone marrow. This type of therapy is intended to partially suppress the bone marrow’s ability to produce new blood cells in order to prevent it from creating new leukemia cells (abnormal white blood cells). 189https://www.ncbi.nlm.nih.gov/pubmed/20130603/ 190https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4366417/ 191http://www.bloodjournal.org/content/93/9/2817?sso-checked=true 192https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4222610/ For instance, in some protocols, the goal is to decrease the leukocyte (white blood cell) count in children with ALL to 1.5 – 3.5×109/L. 193https://www.ncbi.nlm.nih.gov/pubmed/2924255/ 194https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4222610/ While the reference range in children will vary between laboratories, it’s typically around 4.5 – 14.5 109/L for kids between the ages of 6 and 10 years. 195https://www.stjude.org/treatment/patient-resources/caregiver-resources/patient-family-education-sheets/hematology/blood-counts-and-sickle-cell-disease.html 196https://www.childrensmn.org/references/lab/hematology/cbc-reference-value-table.pdf 197http://a1.mayomedicallaboratories.com/webjc/attachments/110/30a2131-complete-blood-count-normal-pediatric-values.pdf
While a reduction in white blood cell count is not uncommon, and may even be a sign of response to therapy when thiopurines are used in IBD, major reductions in white blood cell counts are not the desired outcome. 198https://www.ncbi.nlm.nih.gov/pubmed/11564958 An increase in average red blood cell size (mean corpuscular volume; MCV) as well as a decrease in white blood cell count (WBC) have both been associated in some studies with response to thiopurine therapy in IBD. 199https://www.ncbi.nlm.nih.gov/pubmed/12902847 200https://www.ncbi.nlm.nih.gov/pubmed/12902847 Furthermore, the ratio between MCV and WBC (mean corpuscular volume / white blood cell count) has been used to predict a patient’s response to thiopurine therapy. An MCV/WBC ratio ≥ 12 has been found to correlate to positive outcomes in these cases. 201http://dept.stat.lsa.umich.edu/~jizhu/pubs/Waljee-CGH10.pdf 202https://academic.oup.com/ibdjournal/article-abstract/23/suppl_1/S73/4561381 203https://academic.oup.com/ibdjournal/article-abstract/23/suppl_1/S73/4561381 Reference ranges for both MCV and WBC will vary between laboratories but, in adults, the reference range for MCV is typically 80 – 100 fl (femtoliters), while the reference range for WBC is typically around 4.5 – 11×109/L. 204https://medlineplus.gov/ency/article/003643.htm 205https://labtestsonline.org/tests/complete-blood-count-cbc This means there is a lot of room to hit that MCV/WBC ratio of 12 or more while still having a WBC value that falls within the reference range.
Even so, when Thomas, Jr. and other researchers at the Mayo Clinic looked at the changes in red blood cell size and white blood cell count with thiopurine treatment in IBD, the values seen were still, on average, within normal reference ranges during treatment. Furthermore, for patients who experienced leukopenia (abnormally low white blood cell count), the thiopurine was either discontinued or changed to a lower dose. 206https://www.ncbi.nlm.nih.gov/pubmed/12902847
There are differing approaches to managing potential leukopenia from mercaptopurine or azathioprine use in IBD. A 2018 paper by Warner, et al., “A practical guide to thiopurine prescribing and monitoring in IBD”, published in Frontline Gastroenterology, recommended the following: 207https://fg.bmj.com/content/9/1/10
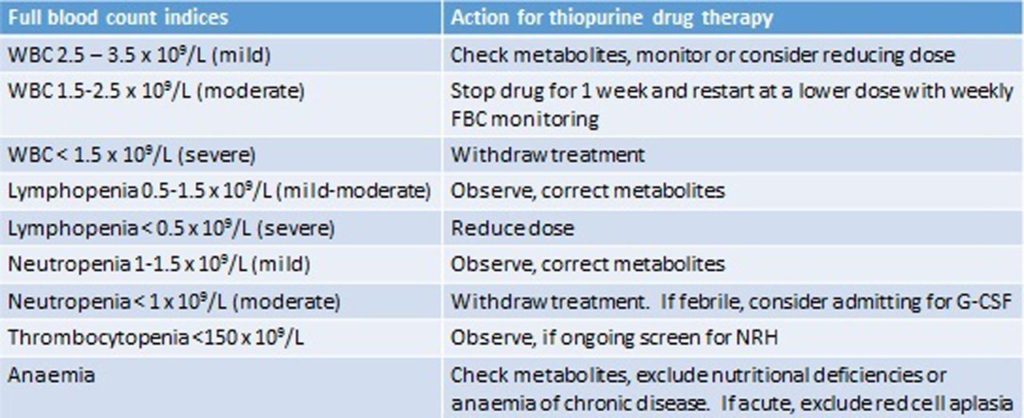
An example of management strategies to resolve abnormal blood laboratory results while a patient is on thiopurine therapy. WBC: white blood cell count. From Warner, et al., published in Frontline Gastroenterology. 208https://fg.bmj.com/content/9/1/10
As you can see, the authors recommend a number of steps, including dose reductions, temporary discontinuations, and complete discontinuation of thiopurine therapy when the WBC value decreases significantly. A temporary discontinuation of thiopurine therapy with dose adjustment and careful monitoring is recommended if a patient’s WBC falls to between 1.5 × 109/L and 2.5 × 109/L; remember that this is actually within the target WBC range in some cancer chemotherapy regimens involving thiopurines. This further underscores the difference between the use of thiopurines as cancer chemotherapy agents and their use as immunomodulators in IBD.
IBD Treatments Incorrectly Labeled “Chemotherapy”

I have seen every IBD medication described as “chemotherapy” at least once; often by patients and bloggers, but also by journalists, healthcare professionals, snake oil peddlers, and various health information websites. By understanding the modern definition of chemotherapy, as covered earlier in this article, it’s fairly easy to determine which medications should not be considered chemotherapy agents. The only true chemotherapy agents commonly used to treat IBD are the ones discussed above (methotrexate, mercaptopurine, and azathioprine), and they’re used as immunomodulators.
The biologics used to treat IBD seem to be mislabeled as “chemotherapy” more than any other class of IBD medication. For this reason, the issues surrounding biologics will be covered in more detail.
Biologics For IBD Are Not Chemotherapy
Generally speaking, a biologic is any medical product made inside a living system or derived from living material. 209https://www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/ucm113522.htm 210https://www.fda.gov/aboutfda/centersoffices/officeofmedicalproductsandtobacco/cber/ucm133077.htm The biologics used to treat IBD are specifically the type known as monoclonal antibodies or therapeutic antibodies. For a brief introduction to what this means, check out my article “What’s in a name?”. The biologics currently approved for use in IBD are: infliximab (Remicade), adalimumab (Humira), certolizumab pegol (Cimzia), golimumab (Simponi), natalizumab (Tysabri), vedolizumab (Entyvio), and ustekinumab (Stelara), as well as any biosimilar versions of these originator biologics.
The idea that the biologic medications used in IBD are chemotherapy agents seems to be an unfortunately common misconception. The biologics used in IBD are not chemotherapy agents, but there is likely a fairly simple explanation for why this idea has persisted so strongly: “chemotherapy administration” billing codes.
The “Chemotherapy Administration” Billing Codes

The American Medical Association (AMA) and the Centers for Medicare & Medicaid Services determine how medical products and services are billed in the U.S.
The Centers for Medicare & Medicaid Services (CMS) and the American Medical Association (AMA) determine the medical billing codes used for all medical services and products in the United States. 211https://www.nlm.nih.gov/research/umls/sourcereleasedocs/current/HCPCS/ 212https://www.cms.gov/Medicare/Coding/MedHCPCSGenInfo/HCPCS_Coding_Questions.html Prior to 2005, the chemotherapy administration billing codes were reserved for “the delivery of antineoplastic agents for cancer treatment” 213http://oig.hhs.gov/oei/reports/oei-09-08-00190.pdf (referring to cytotoxic chemotherapy treatments for cancer). 214https://www.cancer.gov/publications/dictionaries/cancer-terms?CdrID=44816 215https://www.cdc.gov/niosh/topics/antineoplastic/
Around 2005, CMS made changes which has since allowed the chemotherapy administration billing codes to be used for the “parenteral administration of nonradionuclide anti-neoplastic drugs; and also to anti-neoplastic agents provided for treatment of noncancer diagnoses (e.g., cyclophosphamide for auto-immune conditions) or to substances such as monoclonal antibody agents, and other biologic response modifiers.” 216http://oig.hhs.gov/oei/reports/oei-09-08-00190.pdf
Prior to 2005, administration of monoclonal antibody agents like infliximab (Remicade) would have been billed under a lower valued “therapeutic infusion” code 217http://www.hcpro.com/LFS-45403-2459/Medicare-Reform-Advisor-February-8-2005.html (although some medical centers were able to bill for biologic infusions under the chemotherapy administration code prior to 2005, this usage was not officially recognized in CMS policies). 218https://oig.hhs.gov/oei/reports/oei-09-08-00190.pdf But, it’s not as if biologics like infliximab suddenly became chemotherapy drugs around 2005! The guidelines for what could be billed under the chemotherapy administration billing code were simply changed; and, they were changed in order to be more inclusive of medications that, according to CMS, “ . . . [require] physician work and/or clinical staff monitoring well beyond that of therapeutic drug agents [i.e., those billed with the nonchemotherapy administration codes]. . . .” 219http://oig.hhs.gov/oei/reports/oei-09-08-00190.pdf
The monoclonal antibody biologics used to treat IBD are highly complex medications, with a higher requirement for monitoring than less complex, conventional medications. Furthermore, it’s a higher valued billing code compared to non-chemotherapy administration codes, so it’s in a hospital or clinic’s best interest to use it. So, for instance, if a patient receives an infusion of Remicade (infliximab) or Entyvio (vedolizumab) at a hospital or infusion center, they may see it billed to their insurance as “chemotherapy administration” because of the amount of preparation and monitoring that is required of the medical staff (and because it’s a higher valued billing code). Read on to see an actual example of this!
An Example of a Biologic Treatment for IBD Being Billed as Chemotherapy
Below you can see a portion of a Medicare Summary Notice (an overview of products and services billed to Medicare medical insurance). The “Injection, vedolizumab, 1 mg” refers to the biologic vedolizumab (trade name: Entyvio), and the “Infusion of chemotherapy into a vein up to 1 hour” refers to the infusion of vedolizumab that was administered at an infusion center.

A portion of a Medicare Summary Notice with entries for an infusion of vedolizumab (Entyvio) (96413), as well as the vedolizumab itself (J3380).
Here are some important facts about vedolizumab (Entyvio):
- Vedolizumab is a biologic therapy that was developed specifically to treat IBD.
- It is not a cancer treatment.
- It is not cytotoxic/cytostatic.
- Vedolizumab works by inhibiting the migration of white blood cells to the intestines. In doing so, it can help to calm the dysregulated immune response which leads to inflammation. The mechanism of action of vedolizumab does not remotely resemble cytotoxic chemotherapy.
- It is not FDA approved for anything other than IBD (Crohn’s disease and ulcerative colitis). 220http://general.takedapharm.com/content/file.aspx?filetypecode=ENTYVIOPI&CountryCode=US&LanguageCode=EN&cacheRandomizer=71ca4215-8241-4208-97e0-9817e179b45a
It’s important to see that while a biologic used to treat IBD may be associated with a “chemotherapy administration” billing code, this does not mean it is a chemotherapy drug. Remember, a chemotherapy drug is a cytotoxic or cytostatic drug used to treat cancer. Even if you wanted to use the purely literal meaning of “chemotherapy”, “the treatment of disease using chemicals”, it still wouldn’t make sense to call biologics “chemotherapy”. Biologic medications (i.e. monoclonal antibody therapies) are produced from living systems, which is distinctly different from conventional drugs made through chemical synthesis. 221https://dansharpibd.org/whats-in-a-name/ 222https://www.bio.org/articles/how-do-drugs-and-biologics-differ 223https://link.springer.com/chapter/10.1007%2F978-1-4614-6916-2_2
Patients who notice a charge for chemotherapy administration on their insurance may mistake this for meaning their biologic is actually chemotherapy. To make matters worse, some who work in healthcare settings perpetuate this misconception. I’ve heard IBD biologics referred to as chemotherapy by hospital and infusion center employees, and I’ve been told by many IBD patients that employees at their infusion center or hospital told them that their biologic is chemotherapy. For those who work in a medical clinic or hospital, there may be an assumption made that if a service is billed under a chemotherapy administration code, then that means the therapy itself is chemotherapy. Regardless, I think it’s likely that online misinformation has done the most to perpetuate this idea that biologics for IBD are chemotherapy. At particular fault, I believe, are the high-profile health information websites actively spreading this misinformation.
None of the Biologics Approved for IBD are Indicated for Cancer
An additional misconception that I’ve seen in the IBD community is that biologics (monoclonal antibody agents), such as infliximab, are like chemotherapy, only “not as strong”. First of all, comparing therapies based on “strength” is not likely to provide a very accurate or helpful analysis. That said, the overall sentiment can be shown to be misguided no matter how “chemotherapy” is defined. As mentioned previously, biologics are not the same as drugs made through chemical synthesis, so even the erroneous “treatment of disease using chemicals” definition wouldn’t apply. If one considers the most common usage of “chemotherapy”, meaning a cytotoxic/cytostatic treatment for cancer, then the biologics used in IBD would certainly not qualify. The biologics used to treat IBD are not simply “weak” versions of chemotherapy; they are altogether different. Even if one considered the definition of “chemotherapy” to be any medical treatment for cancer, the biologics used to treat IBD still wouldn’t qualify as none of them are indicated for use as cancer therapies. In fact, the biologics (monoclonal antibody agents) that are used to treat cancer tend to work very differently from the biologics used to treat IBD.
The biologics used to treat IBD work by inhibiting pro-inflammatory components of the immune system and by helping to decrease the number of activated immune cells that are contributing to inflammation. 224https://academic.oup.com/ecco-jcc/article/10/8/989/2392174 “Biologics” in cancer treatment can refer to a variety of things beyond just monoclonal antibodies. However, as the general use of “biologic” typically refers to monoclonal antibodies, and since that’s the only type of biologic used to treat IBD, the terms can be considered synonymous for the purpose of this discussion. That said, there are multiple types of biologics used to treat cancer, but they often work by increasing pro-inflammatory components of the immune system or activating cells of the immune system to help them target cancer cells. 225https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/bio-therapies-fact-sheet To help illustrate how diametrically different they can be, consider ipilimumab.
Ipilimumab (Yervoy) is a biologic that is used to treat several different types of cancers. 226https://packageinserts.bms.com/pi/pi_yervoy.pdf Like the biologics used to treat IBD, it is a monoclonal antibody agent (you can tell this by the “mab” at the end of the name). However, while the biologics used to treat IBD work by inhibiting pro-inflammatory components of the immune response, ipilimumab works by blocking part of the immune system’s “off switch”.
Ipilimumab is referred to as an “immune checkpoint inhibitor”. 227https://www.cancer.gov/publications/dictionaries/cancer-terms/def/immune-checkpoint-inhibitor The checkpoint that ipilumumab inhibits is known as CTLA-4 (Cytotoxic T-Lymphocyte Associated protein 4), which is sometimes referred to as an immune system “off switch”. CTLA-4 is an important component of immune system regulation as it helps to control immune responses and prevent one’s immune system from causing self-harm. CTLA-4 is a receptor found on cells of the immune system known as T lymphocytes or T cells. When CTLA-4 receptors on T cells are bound to by other cells of the immune system, it leads to a decrease in immune system activity (in other words, it’s like hitting an “off switch”). 228https://www.ncbi.nlm.nih.gov/gene/1493 Cancer cells may benefit from the activation of this “off switch”, as it prevents the immune system from destroying them. 229https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/bio-therapies-fact-sheet However, when CTLA-4 is blocked, the “off switch” is inhibited, and the immune system will ideally be able to act more freely against cancer cells. 230https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6317697/ 231https://www.cancer.gov/publications/dictionaries/cancer-terms/def/ctla-4 232https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4892769/ 233https://www.ncbi.nlm.nih.gov/pubmed/21930211
The goal of the biologics used to treat IBD have essentially a polar opposite goal to that of ipilimumab. In fact, biologics normally used in IBD, such as infliximab (Remicade) and vedolizumab (Entyvio), have been used off-label to counter an adverse effect that can come from ipilimumab use. An important function of CTLA-4 is to prevent an overzealous immune response that would cause damage to the body’s own tissues. When CTLA-4 is inhibited, such as through the use of ipilumumab to treat cancer, it can lead to immune-mediated inflammation of the digestive tract. As IBD involves immune-mediated inflammation of the digestive tract, it’s easy to see why biologics used to treat IBD might be used to treat this potential adverse effect of ipilimumab. 234https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4913344/ 235https://link.springer.com/epdf/10.1186/s40425-019-0577-1?author_access_token=DCgafkLXh0gVid4swx1jx2_BpE1tBhCbnbw3BuzI2RNaZt6hJmRQEjotPWBLV99lGBel04SguqfH7tlHJAbJSIErSkmaaYQC94ZnA-uLy8L5PZYFj2kHzLKuefAcCRkkhqC3GqZ5rduZ5qMVOTwjVQ%3D%3D 236https://jitc.biomedcentral.com/articles/10.1186/s40425-018-0461-4

Infliximab for the treatment of ipilimumab-induced colitis. An excerpt from the 2016 paper by O’Connor et al., “Ipilimumab-induced colitis: experience from a tertiary referral center”. 237https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4913344/
While biologics used to treat IBD have been used to counter the adverse effects of cancer therapy, none of the biologics used to treat IBD are actually indicated for the treatment of cancer. Feel free to peruse the official prescribing information for all of the originator biologics that are approved for use in IBD:
- Cimzia (certolizumab pegol): https://www.cimzia.com/sites/default/files/docs/CIMZIA_full_prescribing_information.pdf
- Entyvio (vedolizumab): https://general.takedapharm.com/ENTYVIOPI
- Humira (adalimumab): https://www.rxabbvie.com/pdf/humira.pdf
- Remicade (infliximab): http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/REMICADE-pi.pdf
- Simponi (golimumab): http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/SIMPONI-pi.pdf
- Stelara (ustekinumab): http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/STELARA-pi.pdf
- Tysabri (natalizumab): https://www.tysabri.com/content/dam/commercial/multiple-sclerosis/tysabri/pat/en_us/pdfs/tysabri_prescribing_information.pdf
No, Infliximab Was Not A Failed Cancer Treatment
A false claim that has further contributed to the misconception that IBD biologics are chemotherapy is that infliximab (Remicade) was a failed cancer treatment before it became a treatment for IBD. Once again, this claim is being made by large health information websites without corroborating sources or any apparent sense of accountability. Sorting out this claim also provides a good opportunity to further illustrate the often stark difference between approaches used in treating cancer vs. treating IBD.
Computer rendering of the TNF structure 238https://commons.wikimedia.org/wiki/File:TNFa_Crystal_Structure.rsh.png
Infliximab (Remicade) is an anti-TNF monoclonal antibody. TNF is a type of pro-inflammatory cytokine. “Cyto-” means “cell” and “-kine” refers to movement or activity – a cytokine is a cell-derived protein that influences or directs the actions and behavior of cells. 239https://books.google.com/books/about/Love_and_Science.html?id=CycrCQAAQBAJ&printsec=frontcover&source=kp_read_button#v=onepage&q&f=false The cytokine TNF activates inflammatory functions of the immune system. 240https://www.ncbi.nlm.nih.gov/pubmed/10936147 In IBD, the immune system is dysregulated, so there is an overproduction of pro-inflammatory cytokines, like TNF, which leads to chronic inflammation. Infliximab and other anti-TNF biologics used in IBD bind to TNF to block or inhibit its activity in the body in order to calm down the overactive inflammatory response. 241https://www.tandfonline.com/doi/full/10.1586/17512433.2016.1133288 242https://www.nature.com/articles/nri3661
TNF stands for “tumor necrosis factor”, which suggests something that kills tumors. This name was given back in the 1970s following some studies that demonstrated its ability to destroy tumors in mice.243https://www.ncbi.nlm.nih.gov/pmc/articles/PMC433057/ Unfortunately, it was found to be too toxic for use as a cancer treatment in humans. Research on TNF did not stop there, and it was eventually discovered that the inhibition of TNF showed promise as a treatment for autoimmune and immune-mediated conditions. 244https://books.google.com/books/about/Love_and_Science.html?id=CycrCQAAQBAJ&printsec=frontcover&source=kp_read_button#v=onepage&q&f=false 245https://www.ncbi.nlm.nih.gov/pmc/articles/PMC453150/ However, the story of infliximab’s development is a long and winding one. 246https://aadmeetingnews.org/2018-annual-meeting-dailies/the-twisting-journey-from-ca2-to-infliximab/
Infliximab (Remicade) was not even referred to as “infliximab” (let alone “Remicade”) until it had been developed and tested as the anti-TNF treatment we are essentially familiar with today. Before it was given the name “infliximab”, it was known as “ca2”, referring to “chimeric antibody 2” (“chimeric” refers to the fact that the antibody is composed of both human and mouse components). The name “infliximab” would only be applied to a monoclonal antibody that is chimeric and that targets the immune system. The “-li-” portion of “infliximab” refers to the fact that it targets a part of the immune system, the “-xi-” portion refers to it being chimeric, and the “-mab” at the end means it is a monoclonal antibody. You can learn more about how biologics like infliximab are named in my article “What’s in a name? A biologic by any other name would… be a different drug!”.
The development of the chimeric antibody ca2 was based on its precursor, “a2” for “antibody 2”, which was entirely mouse-based. 247https://www.ncbi.nlm.nih.gov/pubmed/8232330 If this precursor version had been released, it’s nonproprietary name could have been something like “inflimomab” (the -o- portion referring to a mouse antibody). Simply put, there is a long history of research and development leading up to the creation of what’s known as infliximab, and the focus had been moved away from cancer long before ca2 became “infliximab”. 248https://www.ncbi.nlm.nih.gov/pubmed/8232330 249https://onlinelibrary.wiley.com/doi/full/10.1111/j.1479-8077.2006.00185.x 250https://aadmeetingnews.org/2018-annual-meeting-dailies/the-twisting-journey-from-ca2-to-infliximab/
Before infliximab (ca2) found success as a treatment for autoimmune and immune-mediated conditions, it was investigated as a treatment for sepsis (due to studies indicating TNF’s role in triggering septic shock). 251https://www.ncbi.nlm.nih.gov/pubmed/3895437/ 252https://www.ncbi.nlm.nih.gov/pubmed/2295861?dopt=Abstract 253https://www.ncbi.nlm.nih.gov/pubmed/3317066?dopt=Abstract It’s absurd that some websites have falsely claimed that infliximab was a failed cancer treatment while failing to mention its history as a potential treatment for sepsis. Unfortunately, ca2 (infliximab) failed as a treatment for sepsis in clinical trials. 254https://www.google.com/search?q=Love+and+Science%3A+A+Memoir&oq=Love+and+Science%3A+A+Memoir&aqs=chrome..69i57j69i60l3.367j0j4&sourceid=chrome&ie=UTF-8 255https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4279876/ 256https://aadmeetingnews.org/2018-annual-meeting-dailies/the-twisting-journey-from-ca2-to-infliximab/ 257https://www.ncbi.nlm.nih.gov/pubmed/7884952 258https://www.ncbi.nlm.nih.gov/pubmed/9734938/ However, ca2 (infliximab) subsequently showed much more success in studies as a treatment for Rheumatoid Arthritis and Crohn’s disease, helping to motivate further research into its use in immune-mediated conditions. 259https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4279876/ 260https://www.ncbi.nlm.nih.gov/pubmed/8250987/ 261https://onlinelibrary.wiley.com/doi/full/10.1111/j.1479-8077.2006.00185.x 262https://aadmeetingnews.org/2018-annual-meeting-dailies/the-twisting-journey-from-ca2-to-infliximab/ 263https://www.gastrojournal.org/article/0016-5085(95)90277-5/pdf 264https://www.ncbi.nlm.nih.gov/pubmed/9321530
The abstract from the 1993 paper by Elliot et al., “Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha.” An important study in the history of infliximab (Remicade). 265https://www.ncbi.nlm.nih.gov/pubmed/8250987/
As you can see, infliximab was not originally developed as a cancer treatment – but its development did eventually occur as a result of research into TNF, which was thought to be a potential cancer treatment. While the idea was originally to treat cancer using TNF, infliximab treats IBD by blocking TNF; polar opposite approaches, but involving the same cytokine known as TNF.
In Cancer, Biologic Is Not Synonymous With Chemotherapy
Biologics (i.e. monoclonal antibody therapies) are used in the treatment of cancer, but they are distinguished from conventional chemotherapy treatments. When used in the treatment of cancer, biologics are typically classified as a type of “immunotherapy” or “targeted therapy”, depending on how they are designed to work. 266http://www.cancer.gov/about-cancer/treatment/types/targeted-therapies 267https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/bio-therapies-fact-sheet
Biologics used to treat IBD are monoclonal antibodies that target and inhibit various pro-inflammatory components of the immune system. As mentioned previously, “biologics” in cancer treatment can refer to a variety of things beyond just monoclonal antibodies. However, as the general use of “biologic” typically refers to monoclonal antibodies, and since that’s the only type of biologic used to treat IBD, that is the only type that will be discussed here. Biologics (monoclonal antibodies) used to treat cancer have different targets than those used to treat IBD, unsurprisingly. For example, they may target and bind to something involved in the cancer’s growth to stop it from becoming active, or they may help the immune system target cancerous cells for destruction. 268https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/bio-therapies-fact-sheet
One biologic that’s used in certain types of cancer, such as non-Hodgkin’s lymphoma, is rituximab (Rituxan). According to the official prescribing information: “RITUXAN is not chemotherapy. […] RITUXAN is a type of antibody therapy that can be used alone or with chemotherapy. They work in different ways to find and attack the cells where cancer starts.” 269http://www.rituxan.com/hem/patient/what-is-rituxan
Some monoclonal antibodies used to treat cancer are actually attached to chemotherapy agents. They are referred to as an antibody-drug conjugate, as they are a combination of a monoclonal antibody (a biologic) with a chemotherapy agent. The idea is that the monoclonal antibody can act as a sort of homing device to deliver a chemotherapy agent more directly to cancerous cells. 270https://www.ncbi.nlm.nih.gov/pubmed/24236714 271http://www.cancer.org/treatment/treatmentsandsideeffects/treatmenttypes/immunotherapy/immunotherapy-monoclonal-antibodies
An example of an antibody-drug conjugate is ado-trastuzumab emtansine (Kadcyla). 272https://www.cancer.gov/publications/dictionaries/cancer-drug?cdrid=564399 According to the manufacturer’s website, ado-trastuzumab emtansine is composed of “2 cancer-fighting drugs in 1”:
- The monoclonal antibody trastuzumab
- A chemotherapy drug 273https://www.kadcyla.com/patient/her2-positive-breast-cancer/how-is-kadcyla-thought-to-work.html
As you can see, there is a distinction made between the monoclonal antibody and the chemotherapy drug that is conjugated to it. To be clear, none of the monoclonal antibodies used in IBD have a chemotherapy agent conjugated to it.
In the article A History of Cancer Chemotherapy, oncologist Vincent T. DeVita Jr, MD, and former president of the American Cancer Society, Edward Chu, MD, had this to say: “The advent of monoclonal antibodies has enhanced the effects of chemotherapy. Hybridomas were described in 1975, and monoclonal antibodies were proven clinically useful starting in the mid-1990s. Although they are not chemotherapy per se, they seem to work best when they are used in conjunction with chemotherapy, as is the case for trastuzumab in breast cancer, cetuximab and bevacizumab in colorectal cancer, and rituximab in non–Hodgkin’s lymphoma, and each are an integral part of chemotherapy regimens for these common tumors.” 274http://cancerres.aacrjournals.org/content/68/21/8643.full
IBD is a Serious Condition
I know that many people with IBD have difficulty gaining understanding and support from people in their lives. Those with IBD are bound to come across people, at some point or another, that minimize the seriousness of their condition – possibly to the patient’s detriment. Telling people “I am on a chemotherapy drug for my IBD” or “they use chemotherapy drugs to treat IBD, too” may help others to understand that IBD is a serious condition requiring serious treatment. I’m certainly not against this, particularly if it’s helping a patient gain some badly needed empathy and support in his or her life. And, it is true that some chemotherapy drugs are also used in the treatment of IBD; but they are suitably classified as immunomodulators when used to treat IBD.
A problem arises, however, when this idea of “chemotherapy for IBD” circulates among patient communities without any additional information on what it means and how the chemotherapy label applies. For instance, a newly diagnosed IBD patient could join any of the hundreds of IBD groups on facebook and come across people referring to treatments they’re considering as “chemotherapy”, or even going so far as to dissuade people from trying a medication because they believe it to be “chemotherapy”. By labeling some or all of the IBD medical treatment options as chemotherapy, we are attaching a lot of preconceived, and very often incorrect, notions about what that term means. Unscrupulous individuals have been known to misapply the “chemotherapy” label to IBD treatments in a deliberate attempt to disinform and cause fear of well-studied medications. This is typically done in order to make non-evidenced-based snake oil seem attractive by comparison.Worse still is the misinformation being propagated by prominent health information websites run by large media companies. These sites are profiting off the spread of harmful misinformation. Despite years of attempting to garner change and accountability on their part, these sites continue to spread such misinformation. Ultimately, I hold them the most accountable, because they have the resources (and medical reviewers) to put out correct information.

Please don’t sue me, Disney.
Something that I advocate strongly for is for people with IBD to make decisions about their health based on accurate information and careful consideration and discussion with one’s medical team; not fear. As a wise, old Jedi master once said,
“Fear is the path to the dark side. Fear leads to anger. Anger leads to hate. Hate leads to suffering.”
-Yoda
A lot of suffering can come from making decisions about one’s health based on fear. The single best thing a person can do to live a healthy, productive life with IBD is to treat it properly. So, if your gastroenterologist suggests a treatment that has a potentially scary-sounding label associated with it, like “chemotherapy”, please try to look past the word and see it for what it is. If you’re a blogger or advocate, please try to encourage other patients to do the same. If you’re a large health information company churning out generally low-quality online content with no apparent sense of accountability – enough, already.
There are potential side effects with any medical therapy. However, the risk of adverse effects of any treatment plan must always be weighed against the very real and significant risks of under-treated or untreated IBD. Any competent IBD specialist gastroenterologist will be able to provide evidence-based information to help patients make the best decisions for their health. Please discuss any concerns you have regarding current or potential treatment plans with your physician.
In Summary
- The term “chemotherapy” is being misused by many in and around the IBD community. As a result, some people with IBD may be making treatment decisions based on faulty information and fear, rather than the best available evidence and careful consideration with a qualified gastroenterologist.
- The term “chemotherapy” was originally coined in 1906 by Dr. Paul Ehrlich to describe his approach to treating infectious diseases. He sought to develop drugs that targeted specific pathogens without harming the host. A more complete name that reflects the original meaning of chemotherapy would be “specific chemotherapy” or chemotherapia specifica.
- Although chemotherapy is sometimes described as “the use of chemicals to treat disease”, this definition is incorrect (both by modern standards and by Dr. Ehrlich’s intended meaning).
- The modern usage of “chemotherapy” almost universally refers to a type of cytotoxic/cytostatic drug used in the treatment of cancer. Several IBD medications (methotrexate, mercaptopurine, and azathioprine) are true chemotherapy agents. However, when they’re used to treat IBD, they are considered immunomodulators. This is a more accurate description of the way they are intended to work in IBD; the dosing of these drugs and the overall treatment plan used in IBD are reflective of this. “Chemotherapy” may also refer to a particular chemotherapy regimen for the treatment of cancer. Cancer chemotherapy regimens are very different from IBD treatment plans.
- The issue with applying the “chemotherapy” label to IBD treatments isn’t because chemotherapy is bad; on the contrary, it’s a very important type of treatment for very difficult diseases (cancers) and the world is absolutely better off with them available. There is a lot of misinformation and fear-mongering regarding the medical treatment of cancer, and too often it leads to people dying of curable diseases. The issue with applying the “chemotherapy” label to IBD treatments is because of the associations people make with the word.
- There are a number of differences between the way chemotherapy agents work in cancer compared to how they work as immunomodulators in IBD. They are typically dosed much more aggressively in cancer in order to produce the desired cancer-killing effects. In addition, chemotherapy agents are used within chemotherapy regimens in the treatment of cancer; this typically involves combinations of cancer-fighting agents, including other chemotherapy drugs. When these drugs are used as immunomodulators for IBD, they are used within an IBD treatment plan.
- The biologics used to treat IBD are not chemotherapy drugs. This is a common misconception which is largely perpetuated by the use of “chemotherapy administration” billing codes applied to the administration of non-chemotherapy agents, such as biologics (monoclonal antibody agents).
- Non-chemotherapy drugs are sometimes mislabeled as chemotherapy by patients, bloggers, healthcare workers, and health information websites. You will likely find that most of the time, it is due to a misuse of the word “chemotherapy”.
- The “chemotherapy” label is also misapplied by unscrupulous people in a deliberate attempt to disinform and cause fear of well-studied medications. This is typically done in order to make non-evidenced-based snake oil seem attractive by comparison. By understanding the information presented here, it should be easier to avoid falling prey to these medical charlatans.
- The single best thing a person can do to live a healthy, productive life with IBD is to treat it properly. So, if your gastroenterologist suggests a treatment that has a potentially scary-sounding label associated with it, like “chemotherapy”, please try to look past the word and see it for what it is.
- Please discuss any concerns you have regarding current or potential treatment plans with your physician.
References





This is an amazing piece of work! I cannot imagine the number of hours you must have spent writing this up, but it is very helpful. I never have heard IBD treatments being called chemotherapy, but I surely appreciate your explanation about the differences especially for Methotrexate, ASA, etc in IBD and then in cancer. Thank you!
Thank you, Annabelle! I really appreciate your kind feedback!
Being involved in an IBD parent support FB group I certainly have seen people being influenced by the misconception about certain IBD meds being called chemo you are talking about. Thank you for this very well done article. It is making the rounds on all the IBD groups I am associated with and will be an excellent resource to share with parents of newly diagnosed children who are being pressured by other people using the word chemo not to listen to their child’s physician.
Thank you for letting me know! Ensuring optimal care for kids with IBD is very important to me, so I’m glad to hear it’s making the rounds in the parent support groups. I plan to make some shareable graphics in the future that will make the info even more accessible for those who don’t have time to read it all.
So much important information here and so well researched. I have definitely heard IBD treatments (specifically biologics) referred to as chemotherapy. This article really clears up that misconception for me. Thanks for your insight!
Thanks, Lisa! I’m glad to know it was helpful!