
Biologic medications are a mainstay of Inflammatory Bowel Disease (IBD) treatment. While the general public is likely only familiar with the trade names of these drugs, such as Remicade and Humira, many patients are also familiar with the generic drug names (a.k.a. INN – International Nonproprietary Names) of these medications. For instance, Remicade’s generic name is Infliximab; Humira goes by Adalimumab.
While the trade names of biologic medications do not necessarily carry any special meaning, the generic/nonproprietary names of biologic medications are anything but arbitrary. The generic/nonproprietary names are mostly determined by how the drug works and how it was made. To put it in a Shakespearean way, a biologic by any other name would be a different drug!
With this article, I will cover the very basic information about IBD biologics that we, as patients, can derive simply by breaking down their names. To begin to understand the names of biologics, it’s necessary to know what a biologic is.
What is a biologic?
A biologic is a medication or product manufactured inside a living system. Biologics are generally composed of large, complex molecules or mixtures of molecules. 1https://www.bio.org/articles/how-do-drugs-and-biologics-differ There are many types of biologic medications and products. For instance, insulin and vaccines are examples of biologics. 2http://www.fda.gov/AboutFDA/Transparency/Basics/ucm194516.htm When speaking of Inflammatory Bowel Disease, any mention of “biologic” is almost certainly referring to the biologic medications used to treat the disease.
Biologics used to treat IBD include: Infliximab (Remicade), Adalimumab (Humira), Certolizumab Pegol (Cimzia), Golimumab (Simponi), Vedolizumab (Entyvio), and Ustekinumab (Stelara).
Biologics are different from conventional chemicals drugs, such as Mercaptopurine (a.k.a. 6MP) and Mesalamine. Conventional chemical drugs have well-defined structures and can be made in a laboratory by combining the individual chemical components of the drug. 3https://www.bio.org/articles/how-do-drugs-and-biologics-differ Biologic medications, on the other hand, are produced inside a living system, and tend to be much more complex and not as easily characterized. 4http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CBER/ucm133077.htm
While there are many types of biologics, the type of biologics presently used in the treatment of IBD are all monoclonal antibodies.
What is a monoclonal antibody?
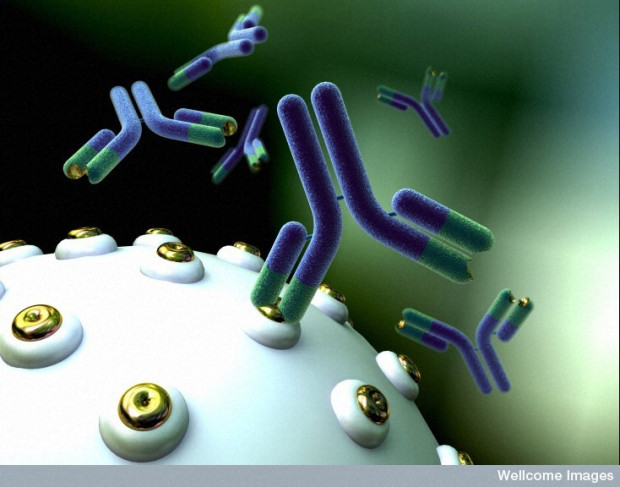
A computer-rendered representation of a monoclonal antibody (the y-shaped structure) binding to an antigen (the gold ring on the white cell surface).
Image credit: Anna Tanczos. Wellcome Images
http://images.wellcome.ac.uk
A monoclonal antibody is “a type of protein made in [a] laboratory that is designed to bind to specific substances in the body”. 5http://www.cancer.gov/publications/dictionaries/cancer-terms?cdrid=46066
They are called an antibody because they bind to and interact with a unique substance, referred to as an “antigen”. 6http://medical-dictionary.thefreedictionary.com/Antibody 7https://www.nlm.nih.gov/medlineplus/ency/article/002223.htm 8http://www.mayoclinic.org/diseases-conditions/cancer/in-depth/monoclonal-antibody/art-20047808
They are monospecific, which means they will only bind to one type of antigen. 9http://www.cancer.gov/publications/dictionaries/cancer-terms?cdrid=46066 10http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3564302/ For instance, anti-TNFα biologics (e.g. Golimumab/Simponi, Infliximab/Remicade, Adalimumab/Humira, Certolizumab Pegol/Cimzia), bind to Tumor Necrosis Factor alpha (TNFα). TNFα is a cytokine that induces an inflammatory response. By binding to TNFα, anti-TNFα biologics can help suppress the inflammatory immune response that takes place in IBD. 11http://www.medscape.com/viewarticle/734142
They are called monoclonal because they are made from a single clone of cells. 12https://www.nlm.nih.gov/cgi/mesh/2011/MB_cgi?mode=&term=Monoclonal+antibodies 13http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3564302/

How do biologic medications get their names?
All medications are given a generic name known as an International Nonproprietary Name (INN). The INN system is administered by the World Health Organization (WHO).
According to the World Health Organization:
“International Nonproprietary Names (INN) facilitate the identification of pharmaceutical substances or active pharmaceutical ingredients. Each INN is a unique name that is globally recognized and is public property. A nonproprietary name is also known as a generic name.” 14http://www.who.int/medicines/services/inn/en/
The INN system “assists in the clear identification, safe prescription and dispensing of medicines to patients; and facilitates communication and exchange of information among healthcare professionals and scientists world-wide.” 15http://www.medsafe.govt.nz/profs/riss/INN.asp#1
While knowing about the INNs of biologics isn’t necessarily that important from a patient perspective, you may find it as interesting as I do. I also think that any accurate insights into IBD and the medications used to treat it can be empowering for patients. It can help to demystify how medications work, or make comprehending other new information easier.
So, without further ado, let’s break down the nomenclature of biologics used in the treatment of IBD.
The meaning behind the names
The names of monoclonal antibodies are composed of four parts:
- The prefix
- substem A (target)
- substem B (species)
- and the suffix.
For example, the generic drug name for Remicade is Infliximab, which, if broken down, would look like: Inf-li-xi-mab. Inf is the prefix, li is the substem A (target), xi is the substem B (species), and mab is the suffix.
Let’s take a look now at the meaning of each part.

Prefix (ex: Infli- in Infliximab): This part of the name does not have to mean anything. It only has to contribute to a unique and euphonious/easily pronounceable name. For instance, any immunomodulating chimeric monoclonal antibody, such as Infliximab (Remicade), could also end in “-liximab”. So, a unique prefix is necessary to differentiate between monoclonal antibodies with the same target and species class. 16http://www.who.int/medicines/services/inn/BioRev2014.pdf?ua=1
Substem A: Target (ex: -li- in Vedolizumab): This part refers to the target class of the antibody. The “target” helps to signify the medication’s intended use. All but one of the biologics that are currently FDA-approved for IBD are of the immunomodulating target class. This is designated by “-lim-“, which can be shortened to “-li-“ or “-l-“ when it makes for a more natural sounding name (although the official designation for this substem is now “-li-” or “-l-” – no longer “-lim-“). Other possible target classes for monoclonal antibodies include bacterial (“-b-” or “-ba-“), tumour (“-t-” or “-tu-“) and interleukin (“-k-“, “-ki-“, or “-kin-“) 17http://www.who.int/medicines/services/inn/BioRev2014.pdf?ua=1
Ustekinumab, which is the most recent original biologic (as opposed to a biosimilar) to be approved for use in IBD (currently, only FDA-approved for Crohn’s disease) 18http://www.healio.com/gastroenterology/inflammatory-bowel-disease/news/online/%7B0b6d8585-0052-4533-807d-64896e5e3e73%7D/fda-approves-stelara-for-crohns-disease, is in the interleukin target class , which is here designated by -kin-.19http://www.who.int/medicines/services/inn/BioRev2014.pdf?ua=1
Substem B: Species (ex: -u- in Adalimumab): This part refers to the species that the antibody is based on. While none of these monoclonal antibodies are manufactured inside a human, they are all based on an actual human antibody form to varying degrees. For example, -u-, as in Adalimumab, signifies a fully human antibody. -Zu-, as in Vedolizumab, signifies a humanized antibody. -Xi-, as in Infliximab, signifies a chimeric antibody. 20http://www.who.int/medicines/services/inn/BioRev2014.pdf?ua=1 All current IBD biologics fall into one of those three categories. Here is what these substems mean:
- Chimeric (-xi-): This antibody is produced by a non-human species, and then modified to resemble human antibodies. The constant domain is human. The variable domain contains human elements, but still resembles that of a non-human. 21http://www.thefreedictionary.com/chimeric 22http://www.who.int/medicines/services/inn/BioRev2014.pdf?ua=1
- Humanized (-zu-): This antibody is produced by a non-human species, and then modified to resemble human antibodies. The constant domain is human. The variable domain mostly resembles that of a human. 23http://www.who.int/medicines/services/inn/BioRev2014.pdf?ua=1 24http://medical-dictionary.thefreedictionary.com/humanized+antibody
- Human (-u-): This antibody may be produced by a non-human species that itself has been genetically modified (e.g. transgenic mice) to produce fully “human” antibodies. 25http://genome.wellcome.ac.uk/doc_wtd021044.html 26http://www.ncbi.nlm.nih.gov/pubmed/7579668 27http://www.ncbi.nlm.nih.gov/pubmed/20811384/ It may also be produced through antibody phage display, which involves genetic engineering of bacteriophages. Adalimumab (Humira) was the first fully human monoclonal antibody to be produced using phage display. 28http://www.nature.com/jid/journal/v134/n2/full/jid2013521a.html
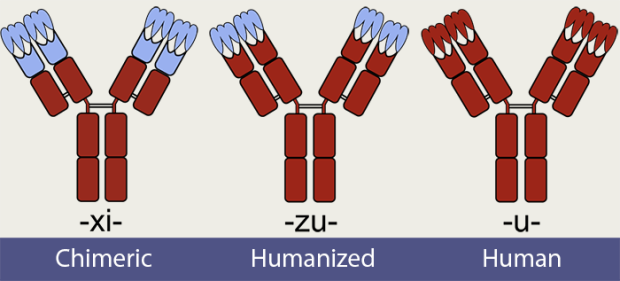
Sketches of chimeric, humanized, and human monoclonal antibodies. The red areas represent human segments, while the blue represents non-human segments. 29https://commons.wikimedia.org/wiki/File:Chimeric_and_humanized_antibodies_with_CDRs.svg

Suffix (ex: –mab in Golimumab): This stands for monoclonal antibody. Monoclonal antibodies are often referred to as mAbs for short. Aw yeah! This part was so easy!
A Second Word
Sometimes, a second word is needed to convey additional information about a biologic. According to the WHO, “if the product is radiolabelled or conjugated to another chemical, identification of this conjugate is accomplished by use of a separate, second word or acceptable chemical designation”. 30http://www.who.int/medicines/services/inn/generalpoliciesmonoclonalantibodiesjan10.pdf
Currently, the only IBD biologic with a second word is Certolizumab Pegol (Cimzia). “Pegol” tells us that the drug has been pegylated. Pegylation means that polyethylene glycol has been bound to the monoclonal antibody. This may be done for reasons such as increasing the half-life of the drug in one’s body. 31http://www.medscape.com/viewarticle/823807_2
Those are the basics behind the INNs, at least as far as the currently available biologics used in IBD are concerned. Now that we are familiar with the elements of names, the following chart may be referred to for information on each drug.
INN Reference Chart for IBD Biologics
This chart covers the very basic information contained within the International Nonproprietary Names (INN) of the biologics used in the treatment of Inflammatory Bowel Disease.
| Drug Name (Trade Name) | Prefix | Substem A (Target) | Substem B (Source) | Suffix | Second Word |
| Infliximab (Remicade) | Inf- | -li-
(immune system) |
-xi-
(chimeric) |
-mab (monoclonal antibody) | N/A |
| Adalimumab (Humira) | Ada- | -lim-
(immune system) |
-u-
(human) |
-mab (monoclonal antibody) | N/A |
| Certolizumab Pegol (Cimzia) | Certo- | -li-
(immune system) |
-zu-
(humanized) |
-mab (monoclonal antibody) | Pegol (antibody is PEGylated – polyethylene glycol attached) |
| Golimumab (Simponi) | Go- | -lim-
(immune system) |
-u-
(human) |
-mab (monoclonal antibody) | N/A |
| Vedolizumab (Entyvio) | Vedo- | -li-
(immune system) |
-zu-
(humanized) |
-mab (monoclonal antibody) | N/A |
| Ustekinumab (Stelara) | Uste- | -kin-
(interleukin) |
-u-
(human) |
-mab (monoclonal antibody) | N/A |
To learn more about International Nonproprietary Names (INN), the World Health Organizations official documents are a great place to start:
International Nonproprietary Names (INN) for biological and biotechnological substances (a review): http://www.who.int/medicines/services/inn/BioRev2014.pdf?ua=1
General policies for monoclonal antibodies: http://www.who.int/medicines/services/inn/generalpoliciesmonoclonalantibodiesjan10.pdf
WHO Drug Information: http://www.who.int/medicines/publications/druginformation/issues/DrugInfo09_Vol23-3.pdf
Essential medicines and health products: International Nonproprietary Names: http://www.who.int/medicines/services/inn/en/
References


